Bio230
This page is dedicated to Bio230 The Evolution of Host-Pathogen Interaction. You can view old syllabi, class webpages and other student work.
Student Webpages on Pathogens
Ebola
Ebola virus disease is a fatal hemorrhagic illness that is a result of Ebola virus infection (Hasan et al., 2019) . The pathogen is a nonsegmented, negative sense, single-stranded RNA virus that belongs to the family Filoviridae and the genus Ebolavirus, There exists five distinct known
species of Ebola virus Zaire, Sudan, Tai forest, Bundibugyo, and Reston. Each of these species differ in their features and fatality (Khalafallah et al., 2017) . Ebola virus disease primarily affects humans and nonhuman primates. Early stage symptoms can include those of other viral diseases
including fever, headache, vomiting, and diarrhea in the early stages. Late stage symptoms include hemorrhagic rash, internal, and external bleeding (Hasan et al., 2019) . Since its detection in 1976, the disease has only been treated by therapeutics that were moderately to slightly successful. Only as of very recently (2019) has a vaccine been approved that has proven to be
effective against Ebola.
Life Cycle
Entry and Fusion-
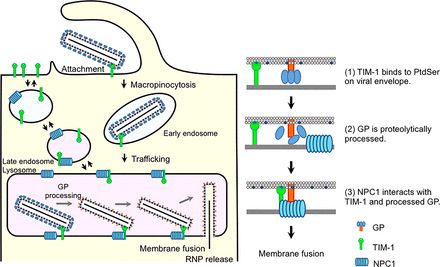
Ebola is spread through direct contact with infected bodily fluids. It invades hosts through breaks in the skin or through mucosal surfaces. This enveloped virus contains anegative-strand RNA genome that encodes for 7 genes and 9 proteins. The glycoprotein, which is
the only surface protein on the viral envelope, is very important in relation to binding and fusion (Menicucci & Messaoudi, 2016) . In the early stages, GP1 interacts with T-cell immunoglobulinand mucin domain 1 receptors. After this binding, the virions enter the host cell through macropinocytosis, a regulated form of endocytosis (Lai et al., 2014 ). The vesicle that takes up
the virus is acidified. The low pH allows EBOV GP to be cleaved by cathepsin, an important process leading to membrane fusion. NPC1 serves as another entry receptor that binds to EBOV GP. This binding results in the conformational changes that trigger membrane fusion (Figure. 2) (Menicucci & Messaoudi, 2016)
As already mentioned, there are many factors that mediate EBOV to host cell attachment. One of those is the interaction of EBOV particles with phosphatidylserine, including TIM. Suppression of some of these individual attachment factors will only partially block host cell attachment. There are numerous factors that play a part in attachment, but the role of NPC1 is
absolutely necessary. In looking at data from Ebola patients over the last 40 years, those with homozygous mutations of NPC1 were shown to be resistant to EBOV infection. This resistance was also seen in NPC1 knockout mice (McElroy et al., 2018)
Transcription and Replication-
EBOV enters the cell cytoplasm as a part of a ribonucleoprotein complex with RNA-dependent RNA polymerase. The RNP is released from the virion so it can serve as a positive complementary stranded RNA. This cRNA serves as the template for the viral genomic RNA (Yu et al., 2017) . The negative sense viral mRNa is also transcribed via the RNA polymerase, capped at the 5’ end with a poly A tail, and subsequently translated. The transcribed EBOV genome consists of NP (nucleoprotein), VP35, VP40, GP (glycoprotein), VP30, VP24 and L protein (polymerase) (Yu et al., 2017) . The VP30 is very important in the initiation of EBOV transcription while VP35 functions as a viral polymerase cofactor (Shabman et al., 2011) . The proteins VP40 and VP24 are associated with the viral lipid coat and are important for virus structure and stability (K. Y. Lai et al., 2014) . Figure 3 provides
a visualization of these processes.
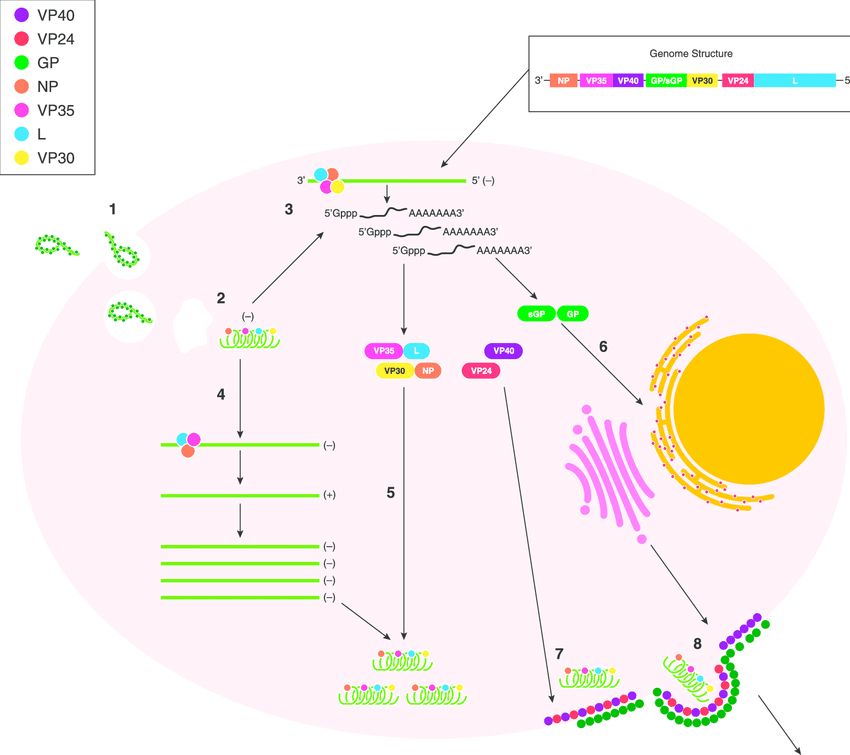
Assembly and Budding-
GP protein is synthesized at the ER, undergoes acylation and other
modifications at the Golgi apparatus. It is then transported to the plasma membrane where it is recruited by the late endosome to meet with VP40 for assembly and budding (Yu et al., 2017) . VP24 has a minor contribution to virion assembly. It has been shown that when the gene for this protein was silenced, there was a reduction in the number of released virions, but replication and transcription are not affected. A detailed understanding of the necessity of VP24 is largely unknown (Yu et al., 2017) . NP forms a nucleocapsid-like structure with VP35 and VP24. It then travels to the cell surface and is incorporated into virions as a result of a NP-VP40 interaction
(Yu et al., 2017) .
There are two major processes involved in the budding of the virion. The first of those being the interaction of VP40 with the inner leaflet of the plasma membrane. It has been observed that VP40 has both electrostatic and hydrophobic components which associate with Phosphatidylserine, a phospholipid component of the cell membrane. VP40 binds to PS, and is able to lock into the cell membrane (Yu et al., 2017) . The other major process is the interaction of GP2 and tetherin. Tetherin is a cell surface protein that can “tether” the virion to the cell membrane, preventing budding (Neil et al., 2008) . GP2 has a glycan cap and a hydrophobic
membrane spanning domain that is thought to play a large role in tetherin antagonisim. (Yu et al., 2017). The exact mechanisms for this, and VP40s interaction with PS are still mostly unknown. Once there is a sufficient amount of negative sense viral genomes and associated viral proteins,
the above process of filoviral budding can occur (Menicucci & Messaoudi, 2016) . Figure 3 illustrates these processes.
Innate Immune Responses
Cytokines and Interferons-
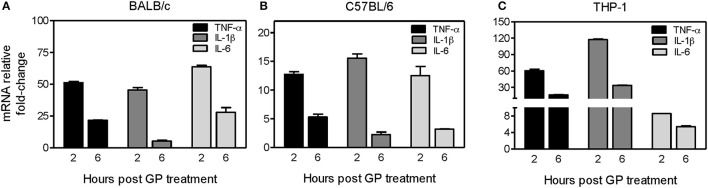
A 2017 study looking at cell cultures infected with Ebola virus showed that GP plays a large part in producing an early immune response upon infection. GP that is shed from virus-infected cells can bind and activate dendritic cells and macrophages leading to the release of pro- and anti-inflammatory cytokines (C.-Y. Lai et al., 2017) . In an analysis of mice and human cells that were exposed to EBOV GP, the induction of pro-inflammatory cytokines TNF-α, IL-1β, and IL-6 increased significantly.
As seen in Figure 4, there was a significant fold increase in these inflammatory cytokines 2 hours after EBOV GP treatment. Transcription returned to basal levels after 24 hours (C.-Y. Lai et al., 2017) . There is evidence that Type I interferons can also play a part in the innate immune response to EBOV infection. A 2007 study showed that infected 293T humans cells that were treated with IFNα were able to inhibit the release of pseudotyped Ebola virions from those cells (Neil et al., 2008) . It was later found that the protein tetherin, which is produced by Type I interferons, was able to restrict viral particle departure with viral infections (Bixler & Goff, 2015) . A caveat to this information is that EBOV proteins VP35 and VP24 have been shown to inhibit the expression and the signaling of Type I interferon genes (He et al., 2019) . Type I IFN levels are relatively high in Ebola infection survivors when compared to the almost nonexistent concentrations in those who die.
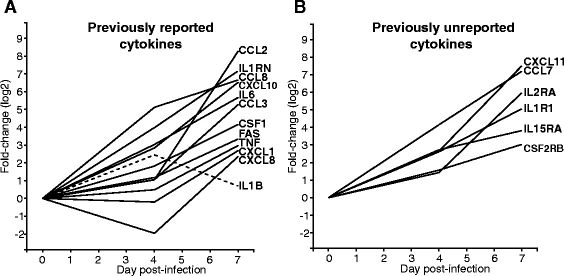
Researchers have also discovered more cytokines that contribute to the inflammatory response. In a study of humans and animals that had been infected with Ebola virus, the serum analyses showed transcriptional upregulations in peripheral blood mononucleated cells by 4 days
post-infection, (IL6, IL1B, IL1RN, CCL8, CXCL10), and by 7 days post-infection (CCL2, CCL3, CSF1, TNF, CXCL1, FAS and CXCL8) (Figure 4A) (Caballero et al., 2016) . Figure 4B also shows 6 other cytokines that follow similar patterns of expression in those infected with ebola virus. At the time of this study, these were cytokines that were not previously known to play a role during Ebola virus infection (Caballero et al., 2016) . Figure 5B also shows 6 other cytokines that follow similar patterns of expression in those infected with ebola virus. At the time of this study, these were cytokines that were not previously known to play a role during Ebola virus infection (Caballero et al., 2016) .
There is not yet a clear consensus on IFNγ and its role in filovirus infections. There is a clear 30 fold increase in IFNγ in the hours after EBOV infection (K. Y. Lai et al., 2014) . With this increase, IFNγ plays a part in the innate immune response by upregulating MHCI and MHCII, thus promoting CD4+ and CD8+ T cell responses (Bixler & Goff, 2015) . However, it also plays a role in the adaptive immune response as it can activate phagocytosis and oxidative burst in neutrophils and macrophages (Bixler & Goff, 2015) . In analyses of Ebola survivors, IFNγ was associated with the activation of cytotoxic T cells and their subsequent viral clearance (Bixler & Goff, 2015) . With this, high levels of IFNγ during infection could promote apoptosis of T cells and disruption to the endothelium. Fatal Ebola infections have shown a loss of T cell-related mRNA expression and DNA fragmentation in leukocytes right after the early production of IFNγ. Figure 6 summarizes the immune response to Ebola infection.
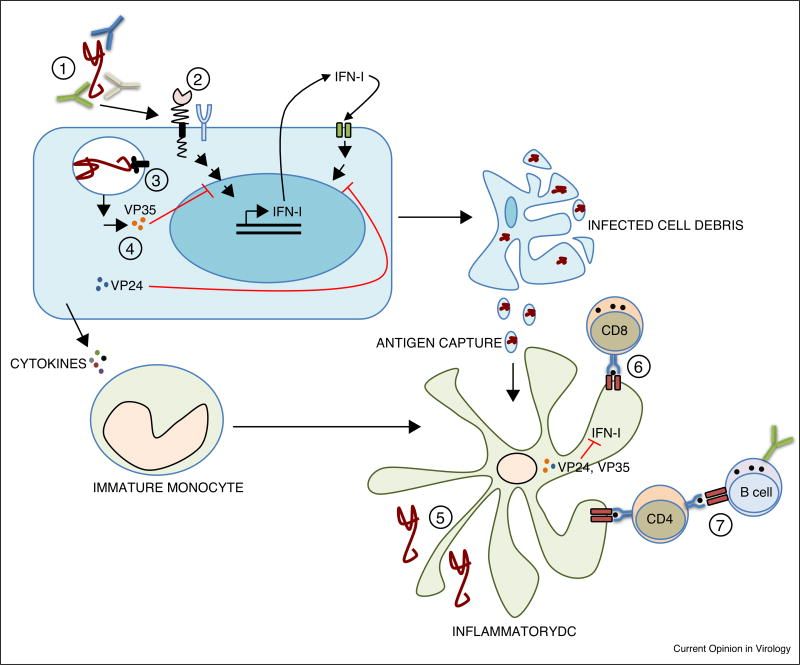
Adaptive Immune Response
Antibodies-
Very little is known about the exact mechanisms of the adaptive immune response to Ebola. In numerous analyses of humans and primates that have been infected with EBOV, high levels of IgG and IgM were detected in survivors. One specific study saw that most of the patients had developed this humoral response 7 days after symptom onset. The increase in antibody concentration corresponded to a progressive reduction in EBOV viremia (Colavita et al., 2019) .
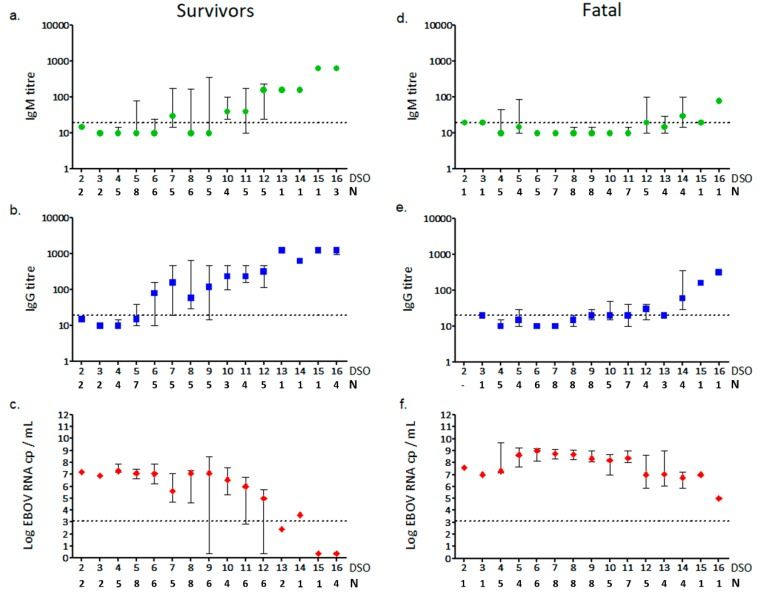
Figure 7 shows a significant difference in the amounts of IgG and IgM in the plasma of survivors vs those who died from Ebola virus. The figure also documents the progression of the viral RNA in the two groups after two weeks. Looking at the data from the fatal cases, these findings suggest that a classic antibody response does not always occur during EBOV infection. The data is also evidence that at least IgG levels may predict an effective control by the host immune system of Ebola virus infection, and could be a marker for a positive progression and outcome from the disease (Colavita et al., 2019) . Scientists are still weary to attribute too much credit to antibodies for favorable patient outcomes. Antibodies are only successful when high
concentrations are able to coat the viron to block infection. With Ebola virus’ many mechanisms of attachment, most of the time the virion is still able to invade the host cell, even if the antibody can successfully bind to the virion. Once in the endosome, antibody access to the virion is very limited and antibody concentrations need to remain high for any antibodies to continue to be attached (Ploquin et al., 2018) . Treatments injecting those infected with Ebola with virus specific antibodies have only shown to be somewhat successful in fighting the disease. It is likely that the mechanisms that Ploquin lays out is the reason for this. The exact role that antibodies play in the immune response against Ebola virus is still very unclear.
T cells-
In an analysis of survivors of survivors of the 2013-2016 West African Ebola virus epidemic, strong data concerning T cell response was found. The study focused on CD8+ T cells, and showed that 96% of the survivors had T cells that responded to the nucleoprotein (NP) present on cells expressing EBOV proteins. Only 38% percent of the survivors had T cells that responded to the two glycoproteins of Ebola (Sakabe et al., 2018) . This finding was unexpected as previous research has suggested that GP is the main target for the immune response.
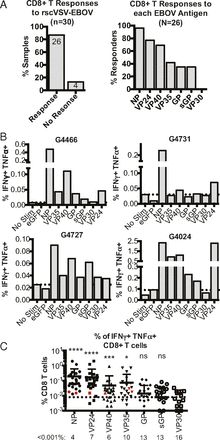
The above figure and the study as a whole gives more insight into the role of CD8+ T cells in clearing Ebola virus infection as part of the adaptive immune response. This research also provides clarity into what exact proteins should be replicated in the development of an effective vaccine against Ebola.
Immune Evasion
VP35-
Ebola virus has been responsible for a number of outbreaks, the majority of them having devastating fatality rates. The lethality of Ebola virus can be attributed to its success in evading human immune responses. The previously described mechanism of macropinocytosis gives Ebola a large advantage when it comes to entry of host cells. The virus uses this nonspecific mechanism while EBOV particles also have the ability to utilize different entry pathways based on both cell type and virus particle size (Aleksandrowicz et al., 2011) . After entry, VP35 has been shown to heavily interfere with the activation of immune responses. One of these interferences is the inhibition of IFN production. VP35 blocks signaling through RIG-I like receptors, preventing the phosphorylation of IRF-3 and IRF-7 (transcription factors for IFNs). This short circuits the IFN response (Messaoudi et al., 2015) . VP35 does this by binding to dsRNA and preventing the RLR-mediated activation of IFN responses. RIG-I then cannot
interact with viral RNAs (He et al., 2019) . VP35 can also interact with the cellular protein PACT, an activator of the IFN-induced antiviral antiviral kinase protein PKR (Messaoudi et al., 2015) . VP35 has also been shown to block the interaction of ΙΚΚε with TBK-1. The ΙΚΚε/TBK-1 interaction also phosphorylates IRF-3 and IRF-7 to produce interferons.
VP24-
VP24 also plays a role in the suppression of the immune response. It can bind to karyopherin-α, a protein that binds to STAT1 to induce the transcription of IFN induced genes (Daugherty & Malik, 2014) . In the absence of VP24, STAT1 is phosphorylated by KPN-α. It can then enter the nucleus to induce interferon stimulating genes. (Ramanan et al., 2011) . Figure 9 shows how VP24 is able to effectively mimic the shape of STAT1 to prevent phosphorylation (Prins et al., 2009) .
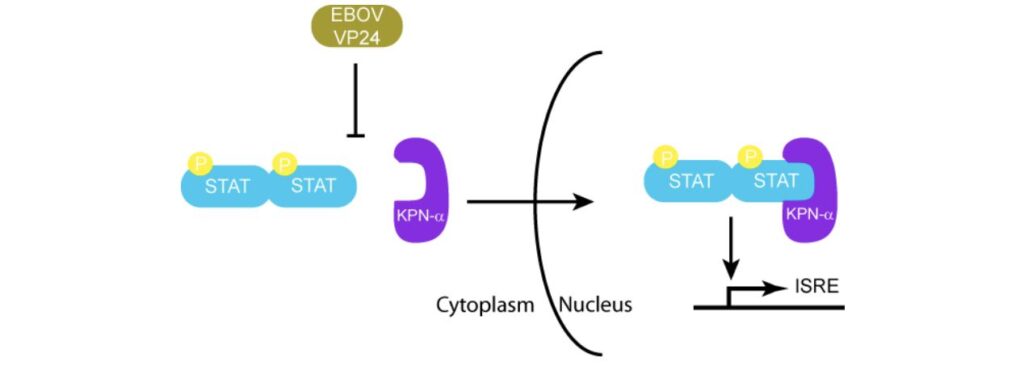
sGP/GP-
Another very interesting phenomenon that may allow immune evasion has been characterized as “antigenic subversion”. In addition to the surface glycoprotein (GP), EBOV also produces a small soluble protein (sGP). A 2012 study looked to see if sGP played some kind of role in interfering with antibodies that were produced against GP. In this study, mice were
immunized with DNA expressing GP and/or sGP to see if antigenic subversion took place.
GP, (D) Immunization with GP, mostly GP specific B-cells can produce antibodies to clear infection before sGP boost from virus (Mohan et al., 2012) .
Figure 10 displays the outcomes of the mice that were immunized with GP and/or sGP. Every group except for one showed a subverted anti-GP response by sGP. The one unsubverted response =occurred when mice were immunized with GP. This group of mice immunized with GP saw the rapid expansion of GP-specific B-cells before an sGP boost. Ebola infection was able to be cleared by the GP antibodies before sGP antibodies were able to interfere. This study provides evidence that the sGP can play a role in immune evasion by Ebola virus by producing “decoys” of antibodies against GP. There is still a lot of research to be done to fully understand this phenomenon. However, this is important information to keep in mind when it comes to vaccine development and providing some protection against Ebola virus.
Treatment-
Since the first identified Ebola outbreak in 1976, there have been a number of approved and experimental treatments against the disease. Some of these include experimental antibody cocktails such as Zmapp and Mab114. The antiviral Remedisivir has also been used on an experimental basis. Very recently, the FDA approved the first treatment for Ebola Virus. This
treatment is called Inmazeb which was developed by Regeneron. Inmazeb consists of three monoclonal antibodies, Maftivimab, Atoltivimab, and Odesivimab. This combination neutralizes Ebola virus by blocking the virus from entering host cells via GP and/or by enabling antibody dependent effector functions ( Inmazeb TM (Atoltivimab, Maftivimab and Odesivimab-Ebgn) Injection , n.d.) .
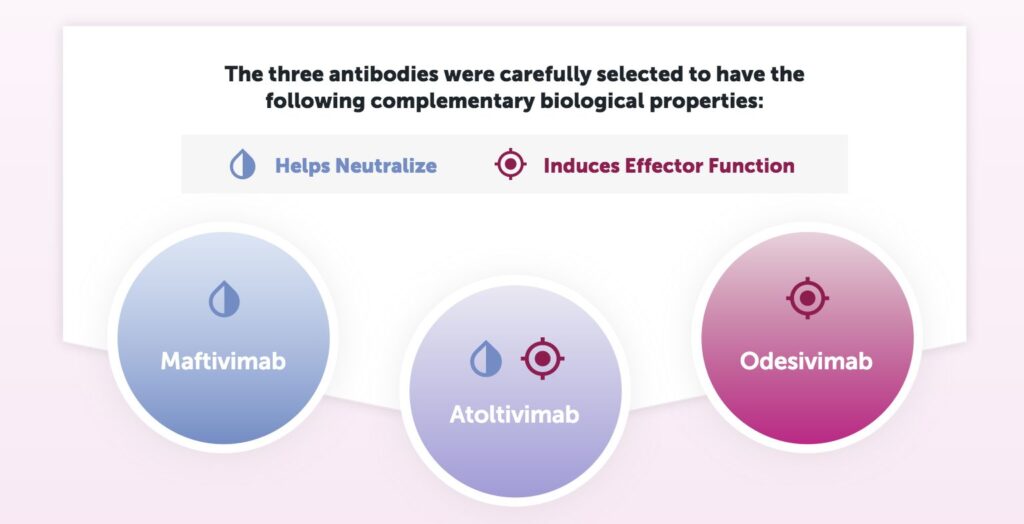
Figure 11 shows the role each antibody plays against Ebola infection. Maftivimab is a neutralizing antibody that blocks entry of the virus into cells. Odesivimab is a non-neutralizing antibody that induces antibody-dependent effector function through FcyRIIIa. Odesivimab also binds to sGP. Atoltivimab has both neutralizing and FcyRIIIa signaling activities (Drugs@FDA ,n.d.) . In the 2018 PALM trial of 307 patients in the Democratic Republic of the Congo, 34% of the patients in the Inmazeb trial died vs 51% of patients in the control trial ( Inmazeb TM ). Each dose was delivered via intravenous infusion. The development and approval of Inmazeb is a very
important step in ensuring the management of future Ebola outbreaks.
Vaccine-
Since the initial 1976 Ebola outbreak in Sudan, many of the same developing nations have suffered from continued outbreaks. This can somewhat be attributed to the lack of a vaccine to provide any protection against Ebola virus. It was only until late 2019 that a vaccine met WHO qualification and FDA approval standards. The recombinant vesicular stomatitis virus–Zaire Ebola virus (rVSV-ZEBOV,), which is sold under the name, Ervebo, is the first vaccine to be approved for the prevention of Ebola virus. The vaccine uses a single recombinant VSV wild type isolate as a vector as shown in Figure 12. The VSV glycoprotein is replaced with Zaire Ebola virus glycoprotein (Medaglini & Siegrist, 2017)
Early studies with mice and macaques found that production of EBOV-GP specific antibodies was the reason for VSV-EBOV-mediated protection (Suder et al., 2018) . The vaccine also showed cross protection against other Ebolavirus species (Suder et al., 2018) . The 2013-2016. Phase 1-3 trials have been conducted since 2014 in countries such as Canada, USA, Liberia, Sierra Leone, Guinea, Gabon, Uganda, and others (Suder et al., 2018) . A study of Ervebo in Guinea during the 2014-2016 outbreak saw 3,537 participants vaccinated. In that trial, thevaccine was shown to be 100% effective in preventing Ebola cases with symptom onset greater than 10 days after vaccination (Henao-Restrepo et al., 2017) . The single dose injection of, VSVZEBOV, produced by Merck, has been shown to be efficacious, immunogenic, and safe. This is an extremely important public health milestone and is very encouraging for future prevention of Ebola outbreaks.
References
Aleksandrowicz, P., Marzi, A., Biedenkopf, N., Beimforde, N., Becker, S., Hoenen, T., Feldmann, H., & Schnittler, H.-J. (2011). Ebola Virus Enters Host Cells by Macropinocytosis and Clathrin-Mediated Endocytosis. The Journal of Infectious Diseases , 204 (Suppl 3), S957–S967. https://doi.org/10.1093/infdis/jir326
Bixler, S. L., & Goff, A. J. (2015). The Role of Cytokines and Chemokines in Filovirus Infection. Viruses , 7 (10), 5489–5507. https://doi.org/10.3390/v7102892
Caballero, I. S., Honko, A. N., Gire, S. K., Winnicki, S. M., Melé, M., Gerhardinger, C., Lin, A. E., Rinn, J. L., Sabeti, P. C., Hensley, L. E., & Connor, J. H. (2016). In vivo Ebola virus infection leads to a strong innate response in circulating immune cells. BMC Genomics , 17 (1), 707. https://doi.org/10.1186/s12864-016-3060-0
Colavita, F., Biava, M., Castilletti, C., Lanini, S., Miccio, R., Portella, G., Vairo, F., Ippolito, G., Capobianchi, M. R., Di Caro, A., & Lalle, E. (2019). Inflammatory and Humoral Immune Response during Ebola Virus Infection in Survivor and Fatal Cases Occurred in Sierra Leone during the 2014–2016 Outbreak in West Africa. Viruses , 11 (4). https://doi.org/10.3390/v11040373
Daugherty, M. D., & Malik, H. S. (2014). How a Virus Blocks a Cellular Emergency Access Lane to the Nucleus, STAT! Cell Host & Microbe , 16 (2), 150–152. https://doi.org/10.1016/j.chom.2014.07.013
Drugs@FDA: FDA-Approved Drugs . (n.d.). Retrieved December 3, 2020, from https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=761169 Ebola (Ebola Virus Disease) | CDC . (2020, November 19).
https://www.cdc.gov/vhf/ebola/index.html
Hasan, S., Ahmad, S. A., Masood, R., & Saeed, S. (2019). Ebola virus: A global public health menace: A narrative review. Journal of Family Medicine and Primary Care , 8 (7), 2189–2201. https://doi.org/10.4103/jfmpc.jfmpc_297_19
He, F. B., Melén, K., Kakkola, L., & Julkunen, I. (2019). Interaction of Ebola Virus with the Innate Immune System. Emerging Challenges in Filovirus Infections . https://doi.org/10.5772/intechopen.86749
Henao-Restrepo, A. M., Camacho, A., Longini, I. M., Watson, C. H., Edmunds, W. J., Egger, M., Carroll, M. W., Dean, N. E., Diatta, I., Doumbia, M., Draguez, B., Duraffour, S., Enwere, G., Grais, R., Gunther, S., Gsell, P.-S., Hossmann, S., Watle, S. V., Kondé, M. K., … Kieny, M.-P. (2017). Efficacy and effectiveness of an rVSV-vectored vaccine in preventing Ebola virus disease: Final results from the Guinea ring vaccination, open-label, cluster-randomised trial (Ebola Ça Suffit!). The Lancet , 389 (10068),505–518. https://doi.org/10.1016/S0140-6736(16)32621-6
Inmazeb TM (atoltivimab, maftivimab and odesivimab-ebgn) Injection . (n.d.). Inmazeb. Retrieved Dec. 3, 2020, from https://www.inmazeb.com
Khalafallah, M. T., Aboshady, O. A., Moawed, S. A., & Ramadan, M. S. (2017). Ebola virus disease: Essential clinical knowledge. Avicenna Journal of Medicine , 7 (3), 96–102. https://doi.org/10.4103/ajm.AJM_150_16
Kuroda, M., Fujikura, D., Nanbo, A., Marzi, A., Noyori, O., Kajihara, M., Maruyama, J., Matsuno, K., Miyamoto, H., Yoshida, R., Feldmann, H., & Takada, A. (2015). Interaction between TIM-1 and NPC1 Is Important for Cellular Entry of Ebola Virus. Journal of Virology , 89 (12), 6481–6493. https://doi.org/10.1128/JVI.03156-14 Lai, C.-Y., Strange, D. P., Wong, T. A. S., Lehrer, A. T., & Verma, S. (2017). Ebola Virus Glycoprotein Induces an Innate Immune Response In vivo via TLR4. Frontiers in Microbiology , 8 . https://doi.org/10.3389/fmicb.2017.01571
Lai, K. Y., Ng, W. Y. G., & Cheng, F. F. (2014). Human Ebola virus infection in West Africa: A review of available therapeutic agents that target different steps of the life cycle of Ebola virus. Infectious Diseases of Poverty , 3 (1), 43. https://doi.org/10.1186/2049-9957-3-43
McElroy, A. K., Mühlberger, E., & Muñoz-Fontela, C. (2018). Immune barriers of Ebola virus infection. Current Opinion in Virology , 28 , 152–160.
https://doi.org/10.1016/j.coviro.2018.01.010
Medaglini, D., & Siegrist, C.-A. (2017). Immunomonitoring of human responses to the rVSV-ZEBOV Ebola vaccine. Current Opinion in Virology , 23 , 88–94. https://doi.org/10.1016/j.coviro.2017.03.008
Menicucci, A., & Messaoudi, I. (2016). Molecular mechanisms of Ebola pathogenesis. Journal of Leukocyte Biology , 100 . https://doi.org/10.1189/jlb.4RI0316-099RR
Messaoudi, I., Amarasinghe, G. K., & Basler, C. F. (2015). Filovirus pathogenesis and immune evasion: Insights from Ebola virus and Marburg virus. Nature Reviews. Microbiology , 13 (11), 663–676. https://doi.org/10.1038/nrmicro3524
Mohan, G. S., Li, W., Ye, L., Compans, R. W., & Yang, C. (2012). Antigenic Subversion: A Novel Mechanism of Host Immune Evasion by Ebola Virus. PLOS Pathogens , 8 (12), e1003065. https://doi.org/10.1371/journal.ppat.1003065
Neil, S. J. D., Zang, T., & Bieniasz, P. D. (2008). Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature , 451 (7177), 425–430.
https://doi.org/10.1038/nature06553
Ploquin, A., Zhou, Y., & Sullivan, N. J. (2018). Ebola Immunity: Gaining a Winning Position in Lightning Chess. The Journal of Immunology , 201 (3), 833–842. https://doi.org/10.4049/jimmunol.1700827 Prins, K. C., Cárdenas, W. B., & Basler, C. F. (2009). Ebola Virus Protein VP35 Impairs the Function of Interferon Regulatory Factor-Activating Kinases IKKε and TBK-1. Journal of Virology , 83 (7), 3069–3077. https://doi.org/10.1128/JVI.01875-08
Ramanan, P., Shabman, R. S., Brown, C. S., Amarasinghe, G. K., Basler, C. F., & Leung, D. W. (2011). Filoviral Immune Evasion Mechanisms. Viruses , 3 (9), 1634–1649. https://doi.org/10.3390/v3091634
Sakabe, S., Sullivan, B. M., Hartnett, J. N., Robles-Sikisaka, R., Gangavarapu, K., Cubitt, B., Ware, B. C., Kotliar, D., Branco, L. M., Goba, A., Momoh, M., Sandi, J. D., Kanneh, L., Grant, D. S., Garry, R. F., Andersen, K. G., Torre, J. C. de la, Sabeti, P. C., Schieffelin, J. S., & Oldstone, M. B. A. (2018). Analysis of CD8+ T cell response during the 2013–2016 Ebola epidemic in West Africa. Proceedings of the National Academy of Sciences , 115 (32), E7578–E7586. https://doi.org/10.1073/pnas.1806200115
Shabman, R. S., Leung, D. W., Johnson, J., Glennon, N., Gulcicek, E. E., Stone, K. L., Leung, L., Hensley, L., Amarasinghe, G. K., & Basler, C. F. (2011). DRBP76 Associates With Ebola Virus VP35 and Suppresses Viral Polymerase Function. The Journal of Infectious Diseases , 204 (Suppl 3), S911–S918. https://doi.org/10.1093/infdis/jir343
Suder, E., Furuyama, W., Feldmann, H., Marzi, A., & de Wit, E. (2018). The vesicular stomatitis virus-based Ebola virus vaccine: From concept to clinical trials. Human Vaccines & Immunotherapeutics , 14 (9), 2107–2113. https://doi.org/10.1080/21645515.2018.1473698
Yu, D.-S., Weng, T.-H., Wu, X.-X., Wang, F. X. C., Lu, X.-Y., Wu, H.-B., Wu, N.-P., Li, L.-J., & Yao, H.-P. (2017). The lifecycle of the Ebola virus in host cells. Oncotarget , 8 (33), 55750–55759. https://doi.org/10.18632/oncotarget.18498

Recent Comments